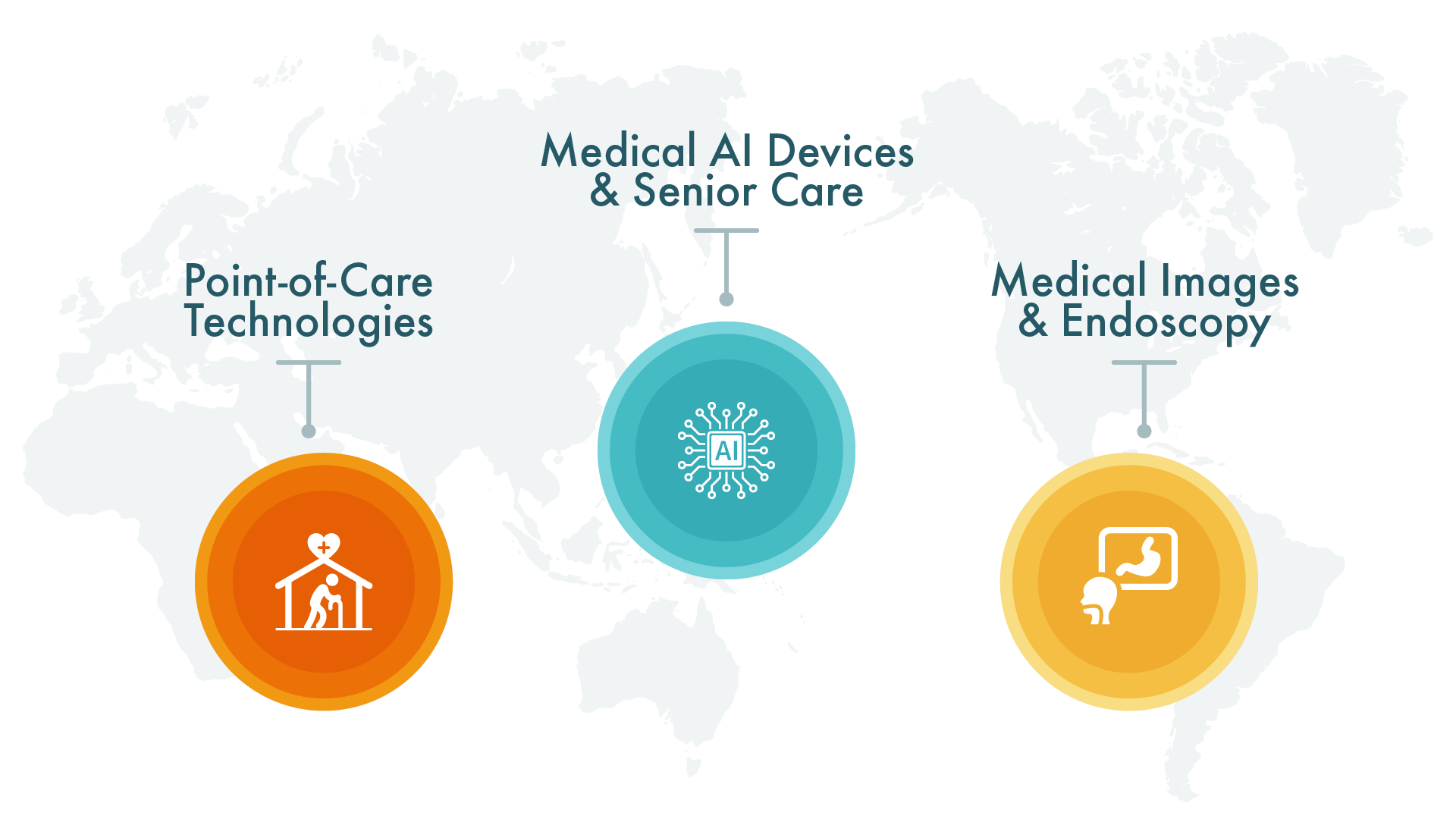
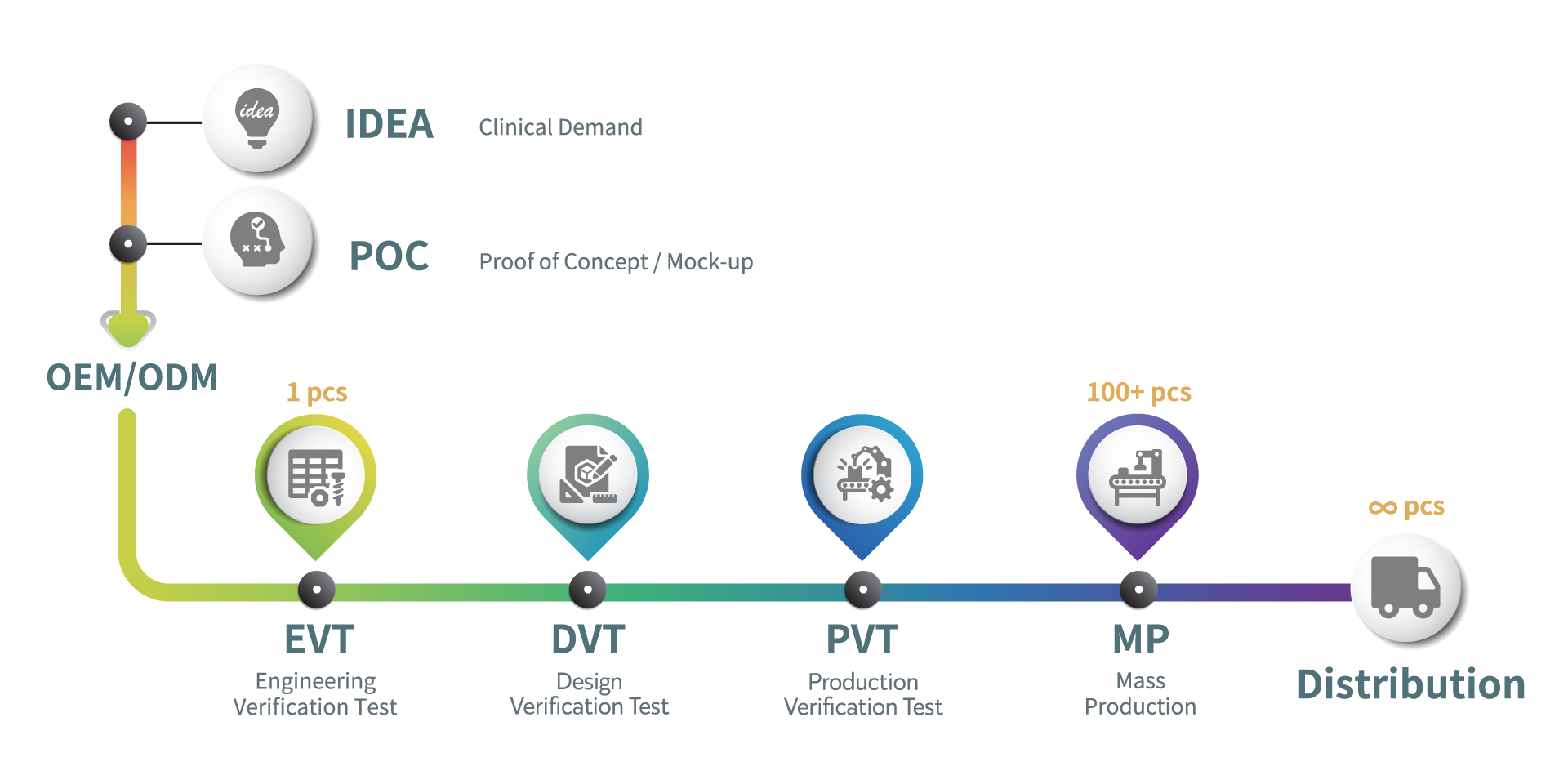
Established in 2013, HUKUI is a distinguished Contract Development and Manufacturing Organization (CDMO) specializing in electronic and medical devices. Leveraging Taiwan’s deep electronic and semiconductor industry expertise and integrated supply chain connections, HUKUI delivers superior Design Engineering Solutions, global regulatory affairs registration services, and a broad spectrum of electronics manufacturing from simple to complex.
HUKUI’s commitment to innovation, quality, and the proven success in delivering comprehensive solutions have made HUKUI the partner of choice for leading medtech companies, meeting the exacting standards of the world’s most advanced products.